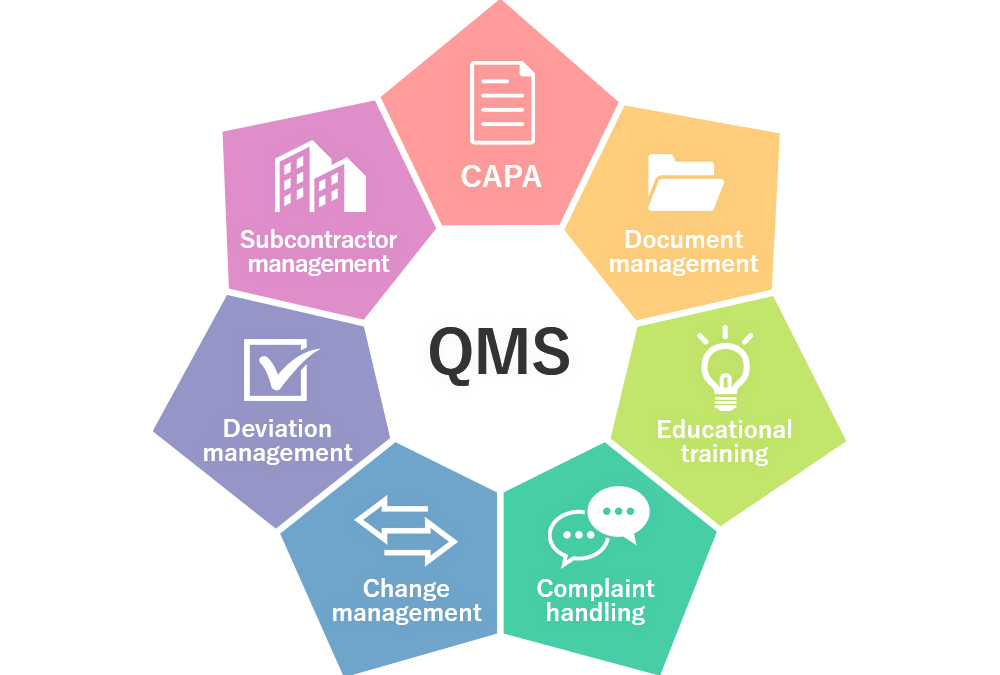
by Elisa Ball | Sep 1, 2022 | Blog, Quality Systems, WDA(H)
Robust Quality Management Systems (QMS) are the heart of our Quality Management. With the right process in place for your business model and the right people managing it, the Quality System will be robust, compliant, easy to navigate and a fantastic tool for trending.
by Jackie (née Heneghan) Peck | Feb 21, 2017 | Blog, CQC, Distance Selling Pharmacy, Falsified Medicines, Good Distribution Practice - GDP, GPhC, MHRA, NHS, Pharmacist, Pharmacy Brands, Pharmacy Suppliers, Quality Systems, Regulation, Training
It is well known that if you tell patients what the side effects of their medicines are then they are much better at complying with the dosage regime. During all consultations, your clinical decisions and patient feedback needs. It should not come as a surprise...
by Jackie (née Heneghan) Peck | Feb 21, 2017 | Blog, Good Distribution Practice - GDP, MHRA, Quality Systems, Regulation, RP, SOPs, WDA(H)
Risk Assessments and Self-audits should cover the whole of your GDP activities during the year. It is best to plan and ensure that you also conduct at least a couple of process audits. Prior to conducting risk assessments, you should look for evidence and discuss with...
by Jackie (née Heneghan) Peck | Feb 13, 2017 | Blog, Bona Fides, Consultancy, CQC, Distance Selling Pharmacy, EudraGMDP, Falsified Medicines, Good Distribution Practice - GDP, GPhC, MHRA, Pharmacist, Quality Systems, Regulation, setting up a pharmacy business, SOPs, WDA(H), WDA(H)
What is the risk of not meeting you suppliers and customers face to face? Call me old fashioned but I like to know who I am dealing with. I like to see the white of their eye, their premises, their procedures. With Valentine’s Day, fast approaching sweethearts are...

by Adel Nagy | Feb 13, 2017 | Blog, Bona Fides, Business, Consultancy, EudraGMDP, Falsified Medicines, Good Distribution Practice - GDP, Humidity, MHRA, Quality Systems, Regulation, SOPs, Transportation, WDA(H)
Having trouble gathering all the necessary information about your customer or supplier? Getting ready for Inspection? Short of time? Having difficulties? PCL have many years of experience in bona fide checks. We offer different levels of help: Bespoke Bona fide Team...
by Ryan Watts | Jul 1, 2015 | Analyisis, Behind the scenes, Blog, Business, Distance Selling Pharmacy, EudraGMDP, External Audit, Good Distribution Practice - GDP, GPhC, MHRA, Pharmacy Suppliers, Quality Systems, Regulation, SOPs, Transportation
In a recent case a supplier of biologically active products for clinical trials was reported to the HSE for not supplying Safety Data Sheets (SDS) to their client. Under the CHIP or Chemicals (Hazard Information and Packaging for Supply) Regulations 2002, suppliers...



